The source of all our energy production is nuclear in nature. The energy of the sun comes from nuclear reactions. Earth heats itself through nuclear reactions which occur deep underground but not enough to sustain life, we need the heat of the sun too.
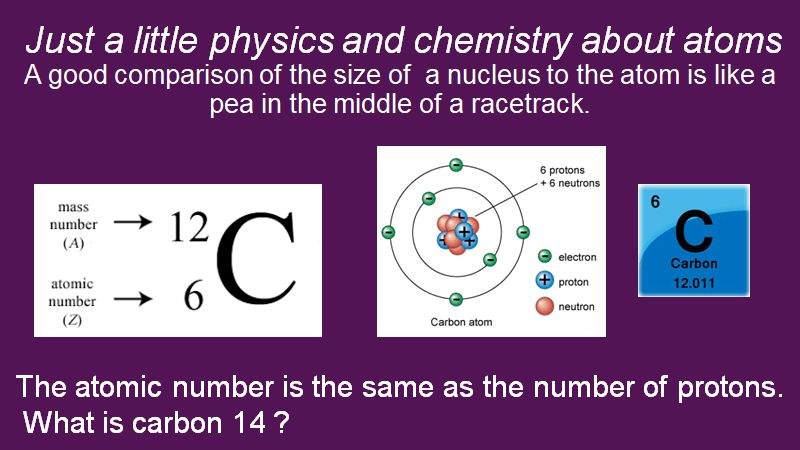
Every element has a unique atomic number, and this number corresponds to the number of protons in its nucleus. Protons are positively charged entities and would push away from each other very quickly if they were not buffered by neutrons. The mass number of an element is simply the sum of its protons and neutrons. Most of the carbon on earth has six protons and six neutrons to a total of twelve.
Carbon 12 is the common isotope of carbon. Carbon-14 still has an atomic number of six, six protons in its nucleus but eight neutrons for a total of 14. Carbon-14 is a radioactive isotope of carbon.
Hydrogen (H) is the lightest element and only has one proton. Deuterium and tritium are isotopes of hydrogen and contain one proton and either one or two neutrons. Thus, tritium has a mass number of three, one proton and two neutrons. The next element with 2 protons is Helium (He).
If, when an atom emits nuclear radiation, the number of protons in the nucleus changes, the atom changes to another element – the element that has the new number of protons.

Uranium is a metal and one of the heaviest, naturally occurring elements on earth. It is ubiquitous in our environment. It is in our drinking water, in our food, in our soils and in ourselves. When any element is much more concentrated in rocks than normal, we refer to it as an ore.
Uranium has 92 protons. Uranium 238 is the most common isotope and makes up 99.3% with 146 neutrons. It is very mildly radioactive with a half-life similar to the age of the earth itself. Uranium 235 only makes up about 0.7% and is much more radioactive. Even rarer, is the uranium 234 isotope with 142 neutrons.
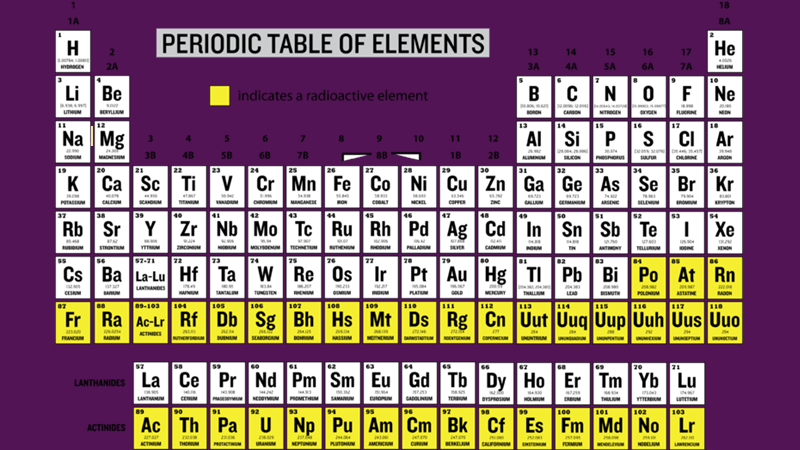
The slide above shows a version of all the known elements. It is hard to see, but hydrogen (H) is right up in the left-hand corner. Uranium (U) is fourth from the left in the bottom row. All the elements on a yellow background are radioactive. Some of the minor isotopes of the elements on the white background are also radioactive such as iodine 131, potassium 40, and carbon-14.
The video below is an excellent presentation on radioactivity. Please watch it! It leads into the next blog. If you have trouble from this site watch it on YouTube:
https://www.youtube.com/watch?v=iTb_KRG6LXo