Australian Associated Press Fri 11 Apr 2025 19.53 AEST https://www.theguardian.com/australia-news/2025/apr/11/science-nerd-ordered-radioactive-materials-parents-sydney-home-spared-conviction-ntwnfb
‘Science nerd’ who ordered radioactive materials to his parents’ Sydney home spared conviction.
A “science nerd” who ordered uranium and plutonium to his parents’ apartment has escaped conviction and been given a two-year good behaviour bond.
Emmanuel Lidden, 24, admitted breaching nuclear non-proliferation laws by ordering various radioactive samples through the internet.
The package’s delivery sparked a major hazmat incident as Australian Border Force officials, firefighters, police and paramedics all combed the scene in August 2023.
But almost two years on, a judge on Friday spared Lidden a conviction and allowed him to walk from Sydney’s Downing Centre district court on a two-year good behaviour bond.
While his actions were criminal, judge Leonie Flannery found that the 24-year-old had mental health issues and displayed no malicious intent.
Speaking outside court after the sentencing, defence lawyer John Sutton said his client was relieved.
But the solicitor criticised border force for the way it had gone after the young man.
“It was an awful investigation for a whole range of reasons,” Sutton said.
Officers overreacted by storming Lidden’s Sydney home in hazmat suits when the amounts ordered were minuscule and harmless, he said.
“We could eat [them] and we’d still be perfectly fine,” he said.
“I’ve been contacted from scientists all around the world saying this is ridiculous.”
Prosecutors should have also questioned whether pursuing the case against Lidden in court was really in the public interest, Sutton said.
In a statement, border force Supt James Ryan called the multi-agency investigation against Lidden “extremely complex and sensitive”.
“The ABF remains committed to protecting the Australian community from all threats which can cross the border,” he said.
“I hope this example can be used as an education tool for people to be aware of the regulatory frameworks around what can and cannot be imported into Australia.”
Lidden is the first person prosecuted under Australia’s non-proliferation laws, aimed at preventing weapons of mass destruction and terrorism.
He ordered the items from a US-based science website and they were delivered to his parents’ home.
He pleaded guilty to two charges – sending nuclear material into Australia and possessing nuclear material.
At a sentence hearing in March, the lawyer described Lidden as a “science nerd” who committed the offences out of pure naivety.
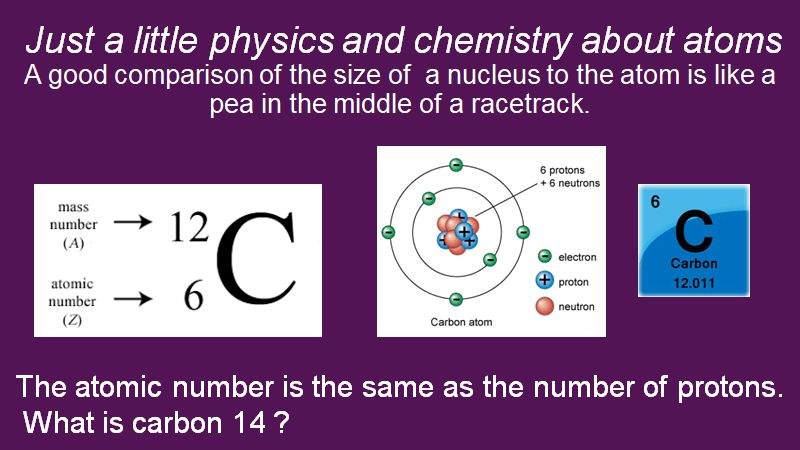
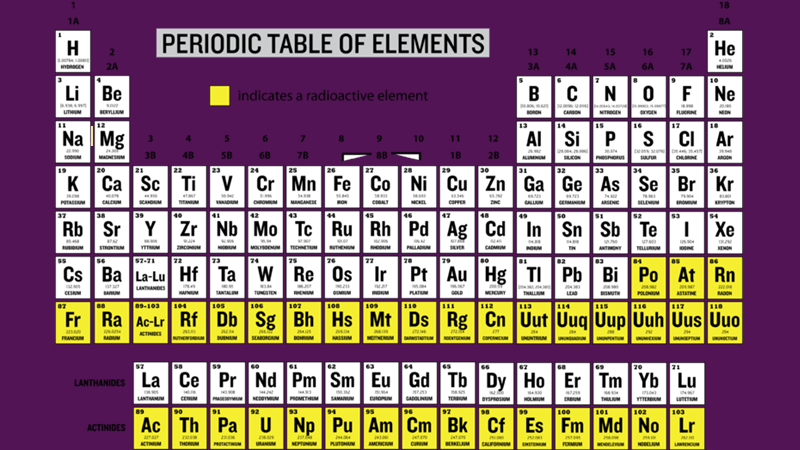
“It was a manifestation of self-soothing retreating into collection; it could have been anything but in this case he latched on to the collection of the periodic table,” Sutton said at the time.
Nuclear materials can be imported legally by contacting the Australian Safeguards and Non-Proliferation Office for a permit first.